Maurizio Benaglia
Università degli Studi di Milano, Italy
Biography
Prof. Maurizio Benaglia has completed his PhD in Organic Chemistry by Università degli Studi di Milano and postdoctoral studies from University of California, San Diego (UCSD) under the supervision of Prof. J. Siegel. In 2001 he won the “Giacomo Ciamician” Medal of the Italian Chemical Society. In 2006 he was promoted to associate professor and in 2015 he became full professor of organic Chemistry at the Department of Chemistry, Univerrsity of Milano. He is author of more than 180 publications on international journals, including four patents, ten review articles and nine book chapters (h index 43). He has been editor of the Wiley book Recoverable and recyclable catalysts (2009).
Abstract
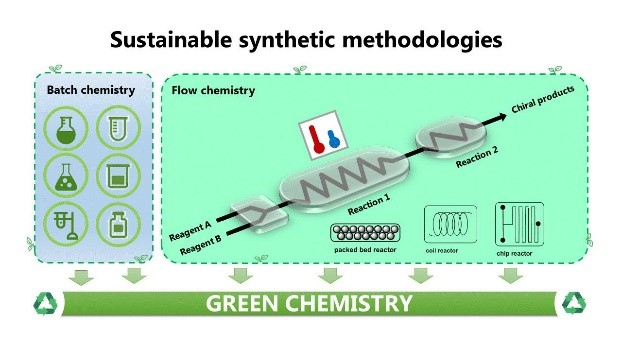
In the synthesis of chiral APIs (active pharmaceutical ingredients) the use of continuous-flow systems are attracting increasing attention.[1] Recently developed technology-assisted stereoselective reactions will be discussed, including reactions of nitroacrylates and catalytic metal-free catalytic reduction of imines to afford chiral, biologically active chiral amines. Some stereoselective transformations have been performed in chiral reactors (packed-bed and monolithic) under continuous flow conditions.[2] Organocatalytic reactions in (micro)-mesoreactors will be also discussed,[3] and compared with stereoselective catalytic in-flow reactions in 3D-printed reactors.[4] The fabrication of ad hoc designed reactors and other devices, to perform at best different reactions becomes now feasible and gives new impulse to the use of enabling technologies in the synthesis of complex molecules.[5]