Conference Schedule
Day1: November 12, 2018
Keynote Forum
Laszlo T. Mika
Budapest University of Technology and Economics
Title: Homogeneous Catalysis in Biomass-Based Solvents

10.00 AM
Biography
László T. Mika received his Ph.D in organic- and organometallic chemistry at Eötvös University, Budapest, Hungary. He works as an associate professor and head of Laboratory of Catalysis at the Budapest University of Technology and Economics and became the head of Department of Chemical and Environmental Process Engineering in 2016. His research activity documented by about 40 scientific papers covers different area of green chemistry (biomass conversion, applications of alternative reaction media, and design new catalytic systems).
Abstract
Solvents are intrinsic part of millions of chemical reactions providing one or more liquid phase(s) and processes such as extraction, filtration, crystallization etc. Thus, the industrial activities using conventional organic solvents are resulting in the release of more than 6 Mt of solvents into the atmosphere in EU-28 annually and some of which are leading to serious environmental concerns. Consequently, the replacement of these conventional organic solvents with green or even renewable-based alternatives could be considered as a key issue in the development of greener and cleaner chemical transformations. The intensive research activities on biomass conversion has led to the identification of novel platform chemicals[i] and some of these could act as alternative non-fossil reaction media for catalysis. It was demonstrated that g-valerolactone as a renewable, polar, aprotic molecule and its ionic liquid derivatives having low vapor pressure even at high temperatures, low toxicity etc. could be applied as biomass-based alternative reaction media for synthetically important homogeneous catalytic transformations such as hydroformylation,[ii]a,b hydrogenation,2c carbonylation2d and cross coupling reactions.[iii] Our contribution will present homogeneous catalysis in biomass-based solvents including comparison of conventional fossil-based media with ones, optimization of reaction conditions, investigation of substrate scopes for corresponding catalytic reactions.
[i] Mika, L. T.; Cséfalvay, E.; Németh, Á. Chem. Rev. 2018, 118, 505.
[ii] (a) Pongrácz, P.; Kollár, L.; Mika, L. T. Green Chem. 2016, 18 (3), 842. (b) Pongrácz, P.; Bartal, B.; Kollár, L.; Mika, L. T. J. Organomet Chem. 2017, 847, 140. (b) Tukacs, J. M.; Novák, M.; Dibó, G.; Mika, L. T. Catal. Sci. Technol. 2014, 4, 2908. (d) Marosvölgyi-Haskó, D.; Lengyel, B.; Tukacs, J. M.; Kollár, L.; Mika, L. T. ChemPlusChem 2016, 81, 1224.
[iii] (a) Orha, L.; Tukacs, J. M.; Gyarmati, B.; Szilágyi, A.; Kollár, L.; Mika, L. T. ACS Sustainable Chem. Eng. 2018, 6, 5097. (b) Strappaveccia, G.; Luciani, L.; Bartollini, E.; Marrocchi, A.; Pizzo, F.; Vaccaro, L. Green Chem. 2015, 17, 1071. (c) Ismalaj, E.; Strappaveccia, G.; Ballerini, E.; Elisei, F.; Piermatti, O.; Gelman, D.; Vaccaro, L. ACS Sustainable Chem. Eng. 2014, 2, 2461.
Matjaz Kunaver
National Institute of chemistry, Ljubljana, Slovenia
Title: Knowledge transfer form research to industry in Adriatic sea area. Case study: Nanocrystalline cellulose from laboratory to pilot plant production

10:30AM
Biography
Assoc.prof.dr. Matjaz Kunaver finished his MSc at the University of Leeds UK in 1991 and has received his PhD degree in 1998 at the University of Leeds UK. He is a senior scientist – researcher at the National Institute of Chemistry, Department for Polymer Chemistry and Technology, Ljubljana, Slovenia and assoc. professor at the University of Ljubljana and Polymer Technology faculty. His main fields of research are the utilization of biomass as a feedstock for polymer synthesis, energy production and nowadays isolation of nanocellulose with new effective methods. He has published more than 56 original scientific papers and 6 patents. He is a member of editorial board of several scientific journals.
Abstract
Waste is a world alarming problem and agricultural and forestry account for ca. 30% of the overall waste produced. The project BIOECO R.D.I. focuses on the idea of the use of biomass from agricultural fisheries and forestry waste and residues with the purpose to raise the green reconversion, multi-functioning, technology innovation, cross-sectoral integration. The project SWOT analysis has identified the main weaknesses affecting enterprises competitiveness in the Adriatic Sea region. All regions have some sort of biomass which could present the opportunity for the region to develop bioeconomy. While the most developed bioeconomy region (Italy) has the biggest potential in agriculture biomass, the rest of regions have more problems with utilization of biomass from agriculture. The reasons vary from legislation limitations to lack of technology to political stand about using fertile land for biomass. One big opportunity is also a biorefinery which is not available in any of the named regions. The problem present in all regions is a transfer of this knowledge from research institutions to industry and integration of solutions to industry. The clusters which could be the catalysts for such cooperation and research driven innovation, are rear in this country. Some countries are trying to adopt the circular change concept, but without bioeconomy that is almost impossible.
Case study: Nanocrystalline cellulose from laboratory to pilot plant production
Method for preparation of NCC by acid hydrolysis in ethylene glycol is a model procedure for NCC isolation from different natural cellulosic sources such as biomass with high yields and products with high crystallinity index. The main advantage of this method is that NCC is in a form of a suspension in organic solvent or water, suitable for further derivatization and functionalization. The process has been transferred to pilot plant production with the capacity of 15 kg of nanocelulose per day. The yields are higher than 45 %, the energy consumption is lower than in the similar processes and the CO2 emissions less than 20% according to the LCA analysis
Tracks
- Green Catalysis | Green Synthesis | Green Solvents | Sustainable Chemistry | Green Organic Chemistry | Biopolymer & Bioplastics | Green Separations | Green Technology | Green Energy | Green Chemistry and Engineering | Green Chemistry in Pharmaceutical Industries
Location: Leonardo Hotel
Laszlo T Mika
Budapest University of Technology and Economics
Chair
Alberto Tagliaferro
Polytechnic University of Turin, Italy
Co Chair
Marcel Risch
University of Goettingen, Germany
Title: The importance of electrochemical materials science for harnessing green energy

11:15-11:40
Biography
Dr. Marcel Risch leads a group at the Institute of Materials Physics at the University of Goettingen. He earned his PhD degree in Experimental Physics at Free University Berlin and subsequently performed postdoctoral work at the Massachusetts Institute of Technology at the electrochemical energy lab. He is enthusiastic about unraveling fundamental relationships between oxide physics and catalytic mechanisms that are relevant to energy storage and conversion processes in electrolyzers, fuel cells and metal-air batteries. Development of operando methods is a cornerstone of his research efforts. He authored 45 manuscripts and his work was cited more than 4200 times in total. His research has previously been awarded with the IAAM Scientist Medal 2018, the Hans-Jürgen-Engell Prize of the ISE and the Carl-Ramsauer Award of the Physical Society of Berlin.
Abstract
The intermittence of green energy such as wind and solar mandates efficient large-scale storage to replace fossil energy. Electrochemical processes enable energy storage and conversion in electrolyzers, fuel cells and batteries, particularly metal-air batteries. A highly fruitful strategy for improving the performance of these devices has been the fundamental insight derived from structure-property relationships of the active materials. Yet, these insights are predominantly based on ex situ investigations. Moreover, the mechanisms of degradation, charge transfer and bond formation are insufficiently understood, which thwarts targeted progress by materials design. More accurate structure-property relationships and elucidation of the mechanisms require innovative spectroscopic and microscopic experiments during reaction conditions. Especially, the making or breaking of the dioxygen bond hinders the efficient production of any sustainable fuel and relates to the stability of electrocatalyst materials as well as battery materials. I will firstly provide an overview on the state-of-the-art structure-property relationships of earth-abundant oxides for the electrocatalysis of oxygen and secondly highlight recent collaborative in situ work using X-ray absorption spectroscopy (XAS), particular using soft X-rays, and environmental electron transmission spectroscopy (ETEM). These experiments provide access to the active state of the materials during reaction conditions and emphasize that the active state can differ drastically from the as-synthesized material. My examples illustrate that surfaces respond dynamically to changing environmental conditions and applied fields. The talk will be concluded with the key challenges for mechanistic insight and an outlook on the future prospects of spectroscopy and microscopy during electrochemical and electrocatalytic processes on defined surfaces.
Jozsef M Tukac
Budapest University of Technology and Economics, Budapest, Hungary
Title: Insight into the activity, selectivity, and stability of heterogeneous catalysts in the continuous flow hydrogenation of levulinic acid and its esters

11:40-12:05
Biography
Jozsef M. Tukacs finished his studies in Organic and Organometallic Chemistry at Budapest University of Technology and Economics, Budapest, Hungary. He works as an assistant professor at the University, in the research team of catalytic processes, led by László T. Mika. It is also apparent from his list of publications. He has published more than 10 papers in reputed journals and he has co-author of a book chapter. He received his papers more than 160 independent references.
Abstract
The global efforts to reduce the carbon dioxide emission and replacement of fossil-based resources demand new and innovative strategies for the green production of fuels, platform molecules and value-added chemicals. The selective conversion of non-edible carbohydrates, e.g. cellulose, chitin, chitosan into platform molecules plays a key role in sustainable development.1 The acid catalyzed dehydration of non-edible carbohydrates results in equimolar formation of levulinic acid (LA) and formic acid.2 Subsequently, the LA and levulinic acid esters (methyl- and ethyl-levulinate) can be converted to γ-valerolactone GVL, which can be considered as a sustainable liquid,3 either by homogeneous,4 or heterogeneous catalyst systems. Recently, the catalytic transfer hydrogenation of LA to GVL in a continuous alternative system was reported.5 The H-Cube® tubular reactor is one of the most promising techniques for the high-throughput heterogeneous catalytic hydrogenation under continuous-flow conditions. Here we report the application of the H-Cube® and H-Cube Pro™ tubular reactors for the hydrogenation of LA and LA esters using environmentally bening solvents such as water and alcohols in the presence of (C6H4-m-SO3Na)2PBu phosphine modified catalyst. We have demonstrated, that the activity of the Ru-based catalyst system can be increased by the application of phosphine ligands6, 7 as well as the LA and LA esters can be selectively and quantitatively converted to GVL in the presence of 5% Ru/C, 5% Pt/C, 10% Pd/C and Raney Ni catalyst and bidentate Ph2P(CH2)nPPh2 (n = 1 – 3) ligands. Details of the reaction conditions, the effect of chelating ring on the activity and the catalyst recycling will be presented.
- Mika, L. T.; Cséfalvay, E.; Németh, Á. Chem. Rev. 2018, 118, 505.
- Szabolcs, Á.; Molnár, M.; Dibó, G.; Mika, L. T. Green Chem., 2013, 15, 439.
- Horváth, I. T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L. T. Green Chem., 2008, 10, 238.
- Tukacs, J. M.; Király, D.; Strádi, A.; Novodárszki, Gy.; Eke, Zs.; Dibó, G.; Kégl, T.; Mika, L. T. Green Chem., 2012, 14, 2057.
- Kopetzki, D.; Antonietti, M. Green Chem., 2010, 12, 656.
- Tukacs, J. M.; Jones, R. V.; Darvas, F.; Dibó, G.; Lezsák, G.; Mika, L. T. RSC Adv., 2013, 3, 16283.
- Tukacs, J. M.; Novák, M.; Dibó, G.; Mika, L. T. Catal. Sci. Technol., 2014, 4, 2908.
Alexander N. Buzynin
Prokhorov General Physics Institute, Russian Academy of Sciences, Moscow, Russia
Title: New Methods of Creating Si and III-V Solar Cells and III-V Detectors

12:05-12:30
Biography
Alexander Buzynin graduated with honors from Moscow State University in 1972. His thesis was devoted to the novel phenomenon of graphoepitaxy, and his 1972 publication with N.N. Sheftal was 6 years ahead of the first American work in this area. Until 1984 Buzynin worked in the Research Institute of Materials Science; from 1984 till present he has been working in the General Physics Institute, Russian Academy of Sciences. The main areas of scientific interests of Prof. Buzynin is research and development of conditions for obtaining structurally perfect single crystals and epitaxial films, development of green semiconductor technologies. More than 150 scientific publications and 40 patents.
Abstract
New weys of creating Si and III-V solar Cells (SCs) and III-V detectors are considered:
(1) Green energy-saving low-temperature technique of p-n junctions fabrication in Si. We developed cheap, low-temperature, energy-saving and environmentally friendly method for forming p-n junctions in silicon for SCs. It is based on the new effect of rapid impurity redistribution in Si through irradiation with low energy (1 ÷ 50 keV) undoped ions (for example Ar +) at low temperatures (<60OC) to form p-n junction. This makes it possible to replace the widely used practice of manufacturing p-n junctions technologies based on high temperature, energy intensive and toxic processes [1,2].
(2) High quality GaAs/Ge/Si structures The development of III–V SCs on Si substrates provides the following: (a) manufacturing of large SCs (300-mm and higher); (b) significant increase in SCs efficiency (in comparison with Si SCs) without significant net cost rise; (c) SCs weight is cut more than in half (specific weight: Si - 2,3; Ge - 5,5; GaAs -5,9 g/cm3), this is especially important in aerospace applications. In order to provide high competitive SCs parameters, it is necessary to obtain high-quality III–V films featuring low density of threading dislocations (not exceeding 106 cm-2). We solved this problem [2,3] .The III-V/Ge/Si structures were used to produce effective III-V solar cells, as well as high-sensitivity THz detectors.
(3) Protective anti-reflective YSZ coatings. The advantages of YSZ coatings for photosensitive devices based on Si, Ge, III-V are: (i) thin mirror-smooth uniform YSZ films have high antireflective, stabilizing, protective and insulating properties, high mechanical strength and abrasion resistance, high chemical and radiation resistance; (ii) YSZ film increases the relative efficiency of SCs on Si, III-V compounds; (iii) SCs life time increases [4].
[1] http://www.spp-j.com/spp/1-1/spp.2014.06A0002
[2] http://dx.doi.org/10.1063/1.4974498
[3] http://www.scirp.org/journal/GSC/
[4]www.intechopen.com/books/advanced-photonic-sciences/fianite-in-photonics
Alberto Tagliaferro
Polytechnic University of Turin, Italy
Title: Waste or resource?. Pyrolyzed products for sustainable smart materials production

12:30-12:55
Biography
Dr. Alberto Tagliaferro is Associate Professor in Solid State Physics at Politecnico Torino. His research activity is mainly on carbon nano and microstructured materials, with particular focus on those from green sources. Raman characterization of such materials and their application in composites, sensors and energy are the main subjects of his current activity He has published more than 160 papers in International Journals and is Associate Editor for BioNanoScience (Springer)
Abstract
Development of industrial age technologies has raised many issues such as depletion of natural resource and environmental pollution. Waste management has became a mandatory problem to solve. However waste streams can also be considered as promising resource to produce new raw materials. Among all the available techniques, thermochemical processes play a great role as they are easily scalable and can be tailored to a wide range of applications. A very attractive thermochemical technique is pyrolysis and in particularly the pyrolytic treatment of lignocellulosic waste that leads to biochar production. Biochar is a cheap and easy-to-tune carbon material that has already found application as material for building insulation2, waste water treatment3, solid fuel4 and electrocatalysis5. It has also been used as filler for composites production to enhance the mechanical and electronic properties. In this presentation, we are showing highlights about the use of biochar from several lignocellulosic waste streams (coffee residues, olive trunks, bamboo cuts) as filler for epoxy resin composites and as conductive carbon material for sensors and electroshielding applications.
- 1 Meadows, Dennis; RANDERS, Jorgan. The limits to growth: the 30-year update. Routledge, 2012.2 Ok, Yong Sik, et al. SMART biochar technology—a shifting paradigm towards advanced materials and healthcare research. Environmental Technology & Innovation, 2015, 4: 206-209.
- 3 Tan, Xiao-fei, et al. Biochar-based nano-composites for the decontamination of wastewater: a review. Bioresource technology, 2016, 212: 318-333.
- 4 Liu, Zhengang, et al. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel, 2013, 103: 943-949.
- 5 Yuan, Yong, et al. Sewage sludge biochar as an efficient catalyst for oxygen reduction reaction in an microbial fuel cell. Bioresource technology, 2013, 144: 115-120.
Matjaz Kunaver
National Institute of Chemistry, Slovenia
Title: Liquefied biomass – A feedstock for adhesives and polyurethanes, a source of nanocellulose and a fuel for gas turbine

13:55-14:20
Biography
Assoc.prof.dr. Matjaž Kunaver finished his MSc at the University of Leeds UK in 1991 and has received his PhD degree in 1998 at the University of Leeds UK. He is a senior scientist – researcher at the National Institute of Chemistry, Department for Polymer Chemistry and Technology, Ljubljana, Slovenia and assoc. professor at the University of Ljubljana and Polymer Technology faculty. His main fields of research are the utilization of biomass as a feedstock for polymer synthesis, energy production and nowadays isolation of nanocellulose with new effective methods. He has published more than 56 original scientific papers and 6 patents. He is a member of editorial board of several scientific journals.
Abstract
Biobased platform chemicals can be provided through lignocellulosic conversion in biorefineries. However to make such a process economical a combination of high value products generation with bioenergy production is essential. Acid catalysed glycolysis of biomass wastes is such an example. This study aims to present some possible pathways how to produce polymers, adhesives, energy and nanocellulose through the same liquefaction reaction. We used the liquefied wood as a polyol in the polyester synthesis since it contains a large number of hydroxyl groups. The polyester was applied in polyurethane foam production with properties suitable for industrial applications. Liquefied biomass reacts with different reactive sites in thermosetting systems containing melamine – urea – formaldehyde resin and was found that a 50% addition of the liquefied wood met the European standard quality demands for particle boards. Two fuels, namely liquefied cotton fibers and liquefied biomass were used in gas turbine. Stable combustion was achieved and experimental results indicate successful utilization of the analyzed biofuels in professional gas turbines.The same liquefaction process was used for the isolation of the nanocrystalline cellulose from biomass. The method is a novelty and is a model procedure for NCC isolation from different natural cellulosic sources with high yields and with high crystallinity index.
Alberto Tagliaferro
Polytechnic University of Turin, Italy
Title: Investigation of carbon nanotubes surface functionalization through Raman and XPS analysis

14.20-14.45
Biography
Dr. Alberto Tagliaferro is Associate Professor in Solid State Physics at Politecnico Torino. His research activity is mainly on carbon nano and microstructured materials, with particular focus on those from green sources. Raman characterization of such materials and their application in composites, sensors and energy are the main subjects of his current activity He has published more than 160 papers in International Journals and is Associate Editor for BioNanoScience (Springer)
Abstract
Currently, the most common way to incorporate carbon nanotubes (CNTs) in polymer matrices, is to functionalize their surface by adding oxygen containing groups. The chemical process that is followed in such occasions, includes strong acids, such as sulfuric (H2SO4) and nitric (HNO3) acid, together with temperatures up to the boiling point of the acids. Moreover, the chemical functionalization procedure can last up to three days, including both the reaction step and the purification that follows after. For this reason and considering the environmental impacts of such a procedure, it is important to investigate the preferable conditions for the functionalization, studying the aforementioned parameters and trying to eliminate the energy, reagents and time consumption. In this framework, an investigation on the chemical functionalization of CNTs has been performed, utilizing X-Ray Photoelectron Spectroscopy (XPS) analysis for the quantification of the oxygen groups attached on the surface of CNTs in each trial, supported by RAMAN spectroscopy that confirmed the preservation of the structural integrity of CNTs, after the treatment. CNTs were prepared via the Catalytic Thermal Chemical Vapor Deposition (CT-CVD) method, using two different catalysts (based on Fe and Co/Mn particles) synthesized in house, in order to investigate also the effect of the structural properties of CNTs in the chemical functionalization effectiveness. A parametric study was carried out, considering the acids concentration, the reaction time and temperature, as well as the CNTs/acid mass/volume ratio. The results revealed that CNTs that have been grown through the Co/Mn catalyst, present smaller diameters and narrow diameter distribution, thus they are more vulnerable to the acidic chemical functionalization in comparison with the CNTs grown on the Fe particles and as a result they can be treated in milder conditions, offering a “greener” chemistry pathway.
Maurizio Benaglia
Università degli Studi di Milano, Italy
Title: Synthesis of chiral molecules: Batch vs flow chemistry.

14:45-15:10
Biography
Prof. Maurizio Benaglia has completed his PhD in Organic Chemistry by Università degli Studi di Milano and postdoctoral studies from University of California, San Diego (UCSD) under the supervision of Prof. J. Siegel. In 2001 he won the “Giacomo Ciamician” Medal of the Italian Chemical Society. In 2006 he was promoted to associate professor and in 2015 he became full professor of organic Chemistry at the Department of Chemistry, Univerrsity of Milano. He is author of more than 180 publications on international journals, including four patents, ten review articles and nine book chapters (h index 43). He has been editor of the Wiley book Recoverable and recyclable catalysts (2009).
Abstract
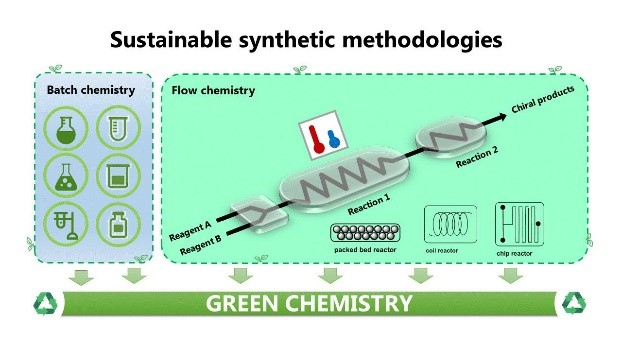
In the synthesis of chiral APIs (active pharmaceutical ingredients) the use of continuous-flow systems are attracting increasing attention.[1] Recently developed technology-assisted stereoselective reactions will be discussed, including reactions of nitroacrylates and catalytic metal-free catalytic reduction of imines to afford chiral, biologically active chiral amines. Some stereoselective transformations have been performed in chiral reactors (packed-bed and monolithic) under continuous flow conditions.[2] Organocatalytic reactions in (micro)-mesoreactors will be also discussed,[3] and compared with stereoselective catalytic in-flow reactions in 3D-printed reactors.[4] The fabrication of ad hoc designed reactors and other devices, to perform at best different reactions becomes now feasible and gives new impulse to the use of enabling technologies in the synthesis of complex molecules.[5]
Fabrizio Olivito
Magna Graecia University of Catanzaro, Italy
Title: Synthesis and pharmacological activity of small organosulfur molecules

15:10-15:35
Biography
Fabrizio Olivito is a PhD student in Life Sciences and technologies attending the last year of PhD. He has obtained a Master’s Degree in Chemistry at the University of Calabria, Italy. He has some experience in organic synthesis with focus on the development of new environmentally friendly synthetic procedures and the synthesis of new bioactive organic compounds. He spent the second year of his PhD at the University of California, Davis at the Chemistry department, where, along with other collaborators, he carried out a project about natural products mimicking, in particular the organosulfur compounds of Allium genus plants, with the assessment of some pharmacological functions. The main goals of his research group are overcome the conventional procedures, avoiding the use of toxic solvent and toxic reagents, especially trying to use water as reaction medium, and open new routes through organic synthesis, going inside structure-activity relationship for drug discovery.
Abstract
Garlic belongs to the Allium genus plants. It has a bewildering number of molecules, and organosulfur compounds are the most representatives. The most known and studied molecule and also the most aboundant is allicine. This molecule is produced from alliin, by the action of the enzyme alliinase that together are released after crashing the garlic bulb. Allicine has many pharmacological activities and there are many papers available in literature about the studies of its properties. One of the main problems of this molecules is the considerable instability. Diallyl disulfide is the most important decomposition product of allicine. Many studies were carried out in the past years about this molecule, regarding biological activity and stability. We recently developed a green procedure to obtain saturated and unsaturated thioacetates starting from organic methanesulfonates. This procedure use water as solvent and avoid the use of a catalyst. Thioacetates are easily hydrolized to thiols and thiols are easily converted to symmetrical disulfides by an oxidation reaction. One of the most important properties of diallyl disulfide is the anticancer activity. Some researchers have recently proved in vitro, the antitumoral activity of this molecule against lung cancer cells with apoptosis and cell cycle arrest. There are many studies that prove that the allylic double bonds play an important role. We synthesized different symmetric disulfides for mimicking this molecule, with double bonds in different positions and with different substituents. We have obtained interesting result in vitro, using A549 lung cancer cells line, that prove that allylic double bond is not the most important driving force, but other factors like substituents or the position of the unsaturation site can affect the activity. We have two new substrates that are quite similar but show higher activity than diallyl disulfide that can open new synthetic routes and studies in this direction.
Qurat Ul Ain Nadeem
University of Liverpool, UK
Title: Fabrication of hybrid nanocoatings for water purification units

15:50-16:15
Biography
Ms. Qurat Ul Ain Nadeem is an active environmentalist who is pursuing her Doctoral research in Environmental Chemistry from Fatima Jinnah Women University, Pakistan and from Stephenson Institute for Renewable Energy, University of Liverpool under a sandwiched scholarship program funded by Higher Education Commission (HEC), Pakistan. Her research advisors include Dr. Rohama Gill and Prof. Dmitry Shchukin, the two renowned names in these particular fields. She has also worked as a research associate in HEC (Pakistan) funded Project No. 5461/Punjab/NRPU/R&D/HEC/2016. She has published quality research works in reputed journals and is working enthusiastically to contribute her energy into the environment of sciences.
Abstract
Water, the most essential entity of life is continuously being adulterated due to the uncontrolled and mismanaged anthropogenic activates and the results are quite destructive. Effective measures are indispensably required to cater the on-growing deterioration of water particularly that of drinking water. Present research is one of the possible greener solutions where mixed-metal oxides and polymer based photocatalytic nanocoatings are designed to treat organic/inorganic pollutants of water. Positive results were made available by synthesizing mixed-metal oxides of ZnO-MgO and ZnO-MnO2 via co-precipitation method followed by their inclusion in multi-layered thin polymeric films using Layer-by-layer (LbL) deposition. The chemical nature and bonding of the prepared ZnO-MgO and ZnO-MnO2 were confirmed by the Fourier-transform infrared spectroscopy (FTIR) analysis while their crystallographic characteristics and phase identification were analyzed by X-ray powder diffraction studies (XPERT-PRO). Prominent IR peaks at 503.44 cm-1 and 472.56 cm-1 along with other characteristic peaks were recorded for ZnO-MgO and ZnO-MnO2 which is within the fingerprint region for both types of mixed-metal oxides. Scherer’s equation was used to calculate the average crystallite size of ZnO-MgO and ZnO-MnO2 which came out to be 29.71 nm and 28.53 nm, respectively. UV-Vis spectrophotometry was utilized to determine the characteristic absorbance of ZnO-MgO and ZnO-MnO2 at 375nm wavelength. Successive deposition of multi-layered thin films for PEI(PSS/ZnO-MgO)n and PEI(PSS/ ZnO-MnO2)n layers was also recorded at 375 nm for 25 bilayer of PEI(PSS/ ZnO-MgO) and PEI(PSS/ ZnO-MnO2), respectively, with increasing absorbance per each layer. The two multilayer systems were built on cellulose acetate membrane filters for designing modular units for industrial and wastewater treatment possessing both photocatalytic degradation and waste water adsorption capabilities.
Mariam Al Saidi
Energy & Building Research Center, Kuwait Institute for Scientific Research (KISR), Kuwait
Title: Synthesis and characterization of N- doped ZnO nanoparticles as efficient photocatalyst and its photocatalytic activity

16:15-16:40
Biography
Eng. Mariam al Saidi is a research assitance of nanotechnology and advanced materials program at kuwait institute for scientific research, kisr since 2016. Eng. Mariam al Saidi has completed her B.E in chemical engineering by Kuwait university. Her research interests include sol-gel chemistry, chemical engineering of photocatalytic processes, design development porous photocatalysts and photocatalytic applications in destruction of organic compounds, hydrogen production. She has participated in few industrial projects in nanotechnology and photocatalysis applications.
Abstract
Preparation of nitrogen- doped zinc oxide (N-ZnO) nanoparticles were carried out by a simple sol-gel method using Zinc acetate and urea precursors at different (1:0, 1; 0.5, 1:1, and 1:2) molar ratios at pH value ~ 7-8. Detailed morphology investigations were characterized using XRD, high-resolution transmission electron microscopy (HRTEM), scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR) and Raman spectroscopy. The methylene blue was chosen as a probe molecule to explore the photocatalytic performance of the prepared photocatalysts compared to that of the commercially ZnO. XRD results confirmed that the synthesized photocatalyst at 1:1 molar ratio was highly crystalline; having hexagonal wurtzite structure. Unlike the 1:2 ratio since it showed much less crystalline structure than the 1:1 mole ratio. The average particle size of best catalyst was estimated to be around 35 nm. The calculations of band gap energy of prepared photocatalysts at 1:1 and 1:2 molar ratios are 3.17 and 3.11 eV, respectively. The photocatalytic performance of the prepared photocatalysts were evaluated by photodegradation of methylene blue where it reaches 97.5% photodegradation efficiency within 30 min for the 1:1 molar ration and 70.42% for the 1:2 molar ratio for 30 min. Also, the calculated reaction rate constant for 1:1 mole ratio is 0.11718 s-1 and for 1:2 is 0.0485 which almost the half of the previous one. The prepared photcatalysts provides an efficient charge carriers transfer with high photocatalytic activity. In addition, the highest photocatalytic efficiency can be explained by large surface area, narrow band gap energy, small particles size, and efficient charge carriers separation.
Youssef Habibi
Luxembourg Institute of Science and Technology, Luxembourg
Title: New biopolymers from renewable building blocks derived from woody hemicelluloses

Biography
Youssef Habibi received his Ph.D. in organic chemistry from Joseph Fourier University (Grenoble, France) prepared jointly with CERMAV (Centre de Recherche sur les Macromolecules Végétales). He is working at the Luxembourg Institute of Science and Technology (LIST) as Lead Scientist. He works across many branches of the sustainable production of materials from renewable resources. His research interests include the design of new bioderived polymers; the development of high-performance nanocomposites from lignocellulosic materials, including natural nanosized fillers; biomass conversion technologies; and the application of novel analytical tools to biomass. He has published over 100 research articles or invited reviews in high-standard peer-reviewed journals, and (co)edited and/or (co)authored several books and book chapters.
Abstract
Lignin is after cellulose the second most abundant biopolymer in the biosphere, and the main polymer based on aromatic units. 50 million tons of lignin are generated every year mainly from wood pulping in the form of sulfonated lignin, but only 2% of this production is used as filler in plastics, as dispersants or for the synthesis of vanillin and DMSO; the remaining is used as energy source. The main limitation to its use as chemical building block is the high content of sulphur moieties, associated with the traditional extraction processes, which impede its reactivity. However, with the emergence sulphur free-lignin classes, there is a renewed interest in exploring the potential of this natural abundant biopolymer, through its conversion by various chemical pathways, toward the production of chemical, additives, fillers, different classes of polymeric materials for a wide range of applications. This keynote presentation will provide an overview of recent advances in converting lignin into functional polymeric materials for more sophisticated applications. An emphasis will be giving to new trends on using sustainable green chemistries in order to produce these new functional polymeric materials, which will open new horizons for this unexploited renewable feedstock.
Youssef Habibi
Luxembourg Institute of Science and Technology
Title: Clicking lignin toward functional materials

Biography
Youssef Habibi received his Ph.D. in organic chemistry from Joseph Fourier University (Grenoble, France) prepared jointly with CERMAV (Centre de Recherche sur les Macromolecules Végétales). He is working at the Luxembourg Institute of Science and Technology (LIST) as Lead Scientist. He works across many branches of the sustainable production of materials from renewable resources. His research interests include the design of new bioderived polymers; the development of high-performance nanocomposites from lignocellulosic materials, including natural nanosized fillers; biomass conversion technologies; and the application of novel analytical tools to biomass. He has published over 100 research articles or invited reviews in high-standard peer-reviewed journals, and (co)edited and/or (co)authored several books and book chapters.
Abstract
Lignin is after cellulose the second most abundant biopolymer in the biosphere, and the main polymer based on aromatic units. 50 million tons of lignin are generated every year mainly from wood pulping in the form of sulfonated lignin, but only 2% of this production is used as filler in plastics, as dispersants or for the synthesis of vanillin and DMSO; the remaining is used as energy source. The main limitation to its use as chemical building block is the high content of sulphur moieties, associated with the traditional extraction processes, which impede its reactivity. However, with the emergence sulphur free-lignin classes, there is a renewed interest in exploring the potential of this natural abundant biopolymer, through its conversion by various chemical pathways, toward the production of chemical, additives, fillers, different classes of polymeric materials for a wide range of applications. This keynote presentation will provide an overview of recent advances in converting lignin into functional polymeric materials for more sophisticated applications. An emphasis will be giving to new trends on using sustainable green chemistries in order to produce these new functional polymeric materials, which will open new horizons for this unexploited renewable feedstock.
Vesna Middelkoop
Flemish Institute for Technological Research - VITO, Belgium
Title: New Frontiers in Greener Catalysis: 3D Printed Chemical Reactors

Biography
Dr. Vesna Middelkoop has an interdisciplinary academic background in materials synthesis, the development of chemical reactors and their advanced characterization. She obtained a PhD degree in the field of synchrotron X-Ray characterization of materials synthesis processes from the University of London in 2010, having worked within the Industrial Materials Group of University College London, Chemistry Department and Birkbeck College, Crystallography Department. She has been working in the R&D industry in London before joining VITO, as a project engineer responsible for the strategic planning and delivery of complex measurement assemblies. Her current research focus is the development of novel structured (and 3D printed) materials for multi-phase chemical reactors (catalysts and adsorbents) within EU-funded and industrial contract research projects.
Abstract
The ordered packing of chemical reactors has found wide application in environmental management (such as carbon structured monoliths for exhaust gas cleaning) and very limited application to date in the chemical industry due to the greater cost of capital investment involved in their manufacture in comparison to other shapes such as granules and beads. As opposed to the conventional randomly packed beds of catalyst bodies, structuring the catalysts into multi-channel reactors and bespoke 3D printed architectures will lead to greatly improved productivity/conversion due to the high surface area and precise and uniform product distribution.
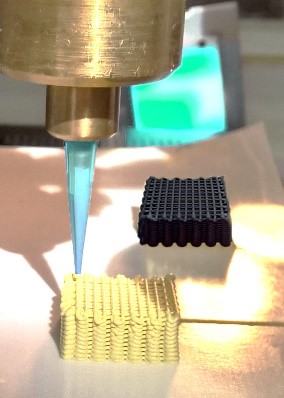
The burgeoning demand for 3D printing technology is due to the method’s suitability as a means of controllable deposition of support and active material in order to produce structured catalyst arrays. The model reactor systems that will be showcased are innovatively employed in industrially relevant chemical reactions. A monolithic multi-channel system was developed using co-printed carbon-supported Pd catalyst to improve organic chemical synthesis. Another system under study is graphene-oxide (GO) based 3D structured catalyst that was produced using a green, rapid, chemical synthesis route combining the unique properties of graphene and active nanocomposite particles for CO2 utilisation reaction.
The initial results of this study on representative reactions are promising as no separation of the catalyst from the product is needed (with no leaching of the catalyst from the support). In addition to catalytic testing in order to extract the catalysts’ pertinent morphological and chemical information and feed it back into the development of the catalyst structures, a combination of conventional characterisation and advanced 3D imaging techniques at multiple resolutions were used.
Noluthando Mayedwa
iThemba Laboratory for Accelerator Based Science, South Africa
Title: Electrochemical, photocatalysis and microbial application of ZnCrO4 nanoparticles prepared via green synthesis using Hibiscus Rosa Sinensis natural extracts

Biography
Dr Noluthando Mayedwa completed her PhD in Chemical Science in 2016 at the University of the Western Cape. Currently appointed as a post-doctoral fellow at iThemba Laboratory for Accelerator Based Science in collaboration with the University of South Africa under the UNESCO/UNISA chair. Her speciality is nanotechnology, electrochemistry, green synthesis, optics, analytical chemistry and energy applications. She is also working on microbial research for application in water treatment.
Abstract
The nanostructures of zinc chromite (ZnCrO4) were synthesized through using Hibiscus Rosa Sinensis natural extracts as an effective bio-reductive and chelating agent. Zinc chromite (ZnCrO4) is a mixed oxide which crystallizes in the cubic system and has a normal spinel structure. The synthesized samples will be characterized by X-ray diffraction (XRD), High resolution scanning electron microscope (HRSEM), High resolution transmission electron microscopy (HRTEM), Energy dispersive X-ray (EDX), diffused reflectance spectroscopy (DRS), photoluminescence spectroscopy (PL) and Fourier transform infrared spectra (FTIR). The nanoparticles will be investigated for photocatalysis application using methylene orange and methylene blue, further studies on microbial activity using different bacterial cultures. The electro activity of ZnCrO4 nanostructures was investigated on glassy carbon electrode, drop coated the binary nanoparticles were systematically studied in alkaline medium of potassium hydroxide (KOH) by cyclic voltammetry (CV), square wave voltammetry (SWV) and electrochemical impedance spectroscopy (EIS).
Biography
Mr. Muhammad Usman, Former Director General of Agricultural Research System, Government of Pakistan who retired from service after a spotless career of about 35 years with senior level experience on research and development of integrated agricultural industries with regard to livestock and dairy development, poultry, aquaculture and apiculture, sustainable agricultural production system, fruits and vegetable, seed production, bioenergy, green chemistry and green energy. Mr. Usman is basically an agricultural scientist with specialization of agricultural/ chemistry working as plant breeder with regard to the yield and quality of various agricultural crops, released several varieties, presented and published research papers on crops and renewable energy in the different conferences like Geneva-Switzerland. Mr. Usman established “Prominent Agro Based Industries SDN BHD” in Malaysia and “Foundation for Rural Development in Pakistan”, with primarily aims to work on integrated agricultural project for Rural Development through improvement in agriculture for rehabilitation of affected area.
Abstract
The aim of presentation consist of green chemistry, health, daily life, crises, poverty and hunger were studied and reported that green chemistry is the major sustainable industry for the development of health, basic need of life, reduce financial crises, poverty and hunger in the world.
Chemistry is the science of composition, structure, properties and reaction of a substance, matter and molecular system. Green chemistry is one of the major and sustainable industry consists of design of chemical products and processes that reduce or eliminate the use or generation of hazardous substance. Green chemistry applies across the life cycle of a chemical product including its design, manufacture, use and ultimate disposal. It is also called as a sustainable chemistry, is an area of chemistry and chemical engineering focused on the designing of products and process that minimize the use and generation of hazardous substances. The major principal of green chemistry including prevention, atom economy, less hazardous chemical synthesis, designing safer chemicals, safer solvents and auxiharies, design for energy efficiency, use of renewable feed stocks etc. In the light of the above study, it is concluded that green chemistry prevent pollution, reduce the negative impact of chemical products, eliminate the amount of toxic substance and minimize the hazards of chemical feed stock, It is the major sustainable industry for the development of health, basic need of life, generate income, increase employment, reduce financial crises, poverty and hunger in the world.
References
- Cernansky, R. (2015). "Chemistry: Green refill". Nature. 519 (7543): 379. doi:10.1038/nj7543-379a.
- Jump up^ Sanderson, K. (2011). "Chemistry: It's not easy being green". Nature. 469 (7328): 18. doi:10.1038/469018a.
- Jump up^ Poliakoff, M.; Licence, P. (2007). "Sustainable technology: Green chemistry". Nature. 450 (7171): 810–812. PMID 18064000. doi:10.1038/450810a.
- Woodhouse, E. J.; Breyman, S. (2005). "Green chemistry as social movement?". Science, Technology, & Human Values. 30 (2): 199–222. doi:10.1177/0162243904271726.
- ^ Jump up to:a b c Linthorst, J. A. (2009). "An overview: Origins and development of green chemistry". Foundations of Chemistry. 12: 55. doi:10.1007/s10698-009-9079-4.
Mohammad Hadi Dehghani
University of Medical Sciences, School of Public Health, Department of Environmental Health Engineering
Title: Experimental Data of Designing an Optimal System for Storage, Collection and Transfer of Household Waste in the GIS Environment, A Case Study of Tehran, IRAN
Biography
Professor Dr. Mohammad Hadi Dehghani (PhD) is a Full Professor at the Tehran University of Medical Sciences (TUMS), School of Public Health, Department of Environmental Health Engineering, Tehran, IRAN. His scientific research interests include the Environmental Science. He is the author of various research studies published at national and international journals, conference proceedings and Head of several research project at the TUMS. He has authored 8 books and more than 150 full papers published in peer- reviewed journals. He is an editorial board member and reviewer in many internal and international journals and is member of several international science committees around the world. He has supervisor and advisor PhD and MSc theses at the TUMS.
Abstract
This study was conducted to correctly manage the system of storage, collection and transfer of wastes in district 22, Tehran. After reviewing existing methods, an optimal system was designed in the GIS environment and appropriate solutions were suggested. Analytical Hierarchy Process (AHP) method was used. After extracting result criteria, these criteria were provided to 15 experts and managers by means of a Delphi questionnaire. Screening of the criteria was done using the criterion importance graph; a necessary condition to apply criteria and sub-criteria, is having at least half the numerical value of each vertical and horizontal vector. The results of the study showed that the most important criterion associated with the selection of waste transfer station is "distance from residential houses" with a final weight of 0.341. "Suitable traffic conditions" and "lack of noise pollution" are the next important criteria with weights of 0.259 and 0.118, respectively. Finally, "non-destruction of recreational facilities" was chosen as the least important (weight of 0.03). Transfer in this district is also 100% mechanized. At the district level, there are 10 garbage trucks, of which 7 collect during night and 3 during day. Given per capita of the district, it takes about 10 minutes to collect each ton of waste. In general, in order to investigate and plan specific methods in the study district, using Geographic Information System, the location of reservoirs in residential and commercial districts has been determined and suggested with a coefficient of 0.75.
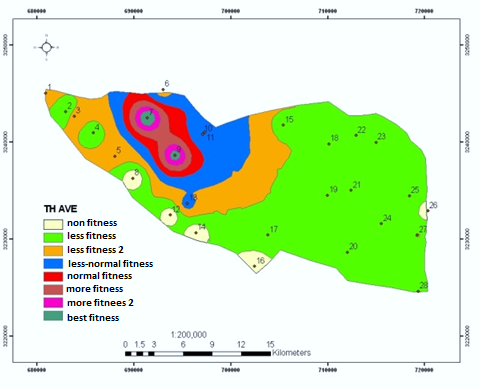
Fig. Prioritization of optimal locating criteria for the urban waste transfer station.
Day2: November 13, 2018
Keynote Forum
Tracks
- Green Solvents | Green Sustainable Agriculture | Green Synthesis | Green Technology | Green Energy | Pollution prevention | Renewable & Recyclable Materials | Sustainable Chemistry | Waste Management | Future Trends in Green Chemistry
Location: Leonardo Hotel
Wei Zhang
University of Massachusetts Boston, USA
Title: Green synthesis of heterocyclic compounds and asymmetric organocatalysis

Biography
Prof. Wei Zhang is the Director of the Center for Green Chemistry at the University of Massachusetts Boston. He had positions of Research Assistant Professor at the University of Pittsburgh, Senior Chemist at DuPont Agricultural Products, and Director of Discovery Chemistry at Fluorous Technologies, Inc. (FTI). His research is in the areas of green chemistry, fluorous chemistry, synthetic free radicals, organocatalysis, and medicinal chemistry. He has published over 180 peer-reviewed papers including three Chem. Rev. and four Tetrahedron Reports, and a book “Green techniques for organic and medicinal applications” (Willey 2012 & 2017). He is currently severing as editor and editorial advisory member on seven international journals including ACS Combinatorial Science and Green Processing and Synthesis. He received the International Fluorous Technology Award in 2015.
Abstract
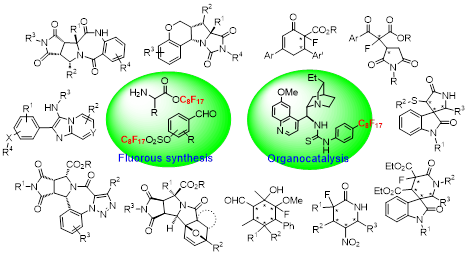
This presentation highlights our recent effort on the development of fluorous chemistry based pot-economic synthesis and asymmetric catalysis to maximize reaction and separation efficiency in the synthesis of diverse heterocyclic scaffolds with substitution, skeleton, and stereochemistry variations. Fluorous recyclable organocatalyst-promoted cascade reactions have been introduced for asymmetric fluorination, Michael addition, Mannich reaction, Robinson annulation and other transformations to construct drug-like molecules with multiple stereocenters. Screening of compounds for druggable targets such bromodomains, kinases, RORgt, and HIV-1 will be mentioned.
Vitaliy Budarin
University of York, UK
Title: Novel green technologies : bio-waste conversion to added value products

Biography
Dr .Vitaliy Budarin graduated from Kiev State University (Ukraine) with an MSc (1983) and PhD (1988) in Chemistry. Following postdoctoral research at Kiev State In 1996 he was appointed to a Lectureship in Chemistry at Kiev State University and was promoted rapidly to Associate Professor in 1998. In 2002 he moved to the Green Chemistry Centre of Excellence (GCCE. From 2012 he became Head of the Microwave technology platform group. From 2017 he is Head of Mesoporous Materials TP and the GCCE’s Principle Scientist. Dr. Vitaliy Budarin experience has led to more than 140 research papers in peer reviewed journals, three patent, four patent applications and five book chapters. Dr. Budarin’s h-factor is 33.
Abstract
It has been shown that two novel green technologies (Low temperature microwave activation and Starbon® materials preparation) could be efficiently applied for bio-waste utilisation. Combination of these processes could help transfer polysaccharide reach biomass to high value products such as fuels, chemicals, materials and solvents. Both technologies are scalable and could be applied for multi-tonne processes. The microwave (MW) technology focuses on depolymerisation of large organic molecules to high value products. The most promising from the industrial application perspectives is ability of MW irradiation to activate lignocellulosic materials at very low temperature. In inert atmosphere MW assisted pyrolysis could produce a number of high value chemicals such as levoglucosan, levoglucosenone, HMF and furfural. The grade yield and high purity of these products are guaranteed by low temperature of the process and high controllability of microwave irradiation. In the presence of water solution the lignocellulosic biomass could be converted to different types of mono- and oligosaccharide. This sugars-reach solution could be used for further biological/enzymatic treatment and production of bio-gas and bio-ethanol. Starbon® technology is complimentary to microwave approach helping to convert helical-structured polysaccharides (starch, pectin, alginic acid and xylan) to mesoporous carbonaceous materials. Starbon® materials due to its textural properties flexibility and controllable functionality could be applied for recovery and purification of critical metals such as lithium, cobalt, beryllium, silver and gold from aqueous systems. High degree of mesoporosity and large pore diameter (larger than 5nm) of Starbon® enables to perform reversible adsorption of bulky industrial days.
Sami Fadlallah
Unité Matériaux et Transformations (UMET), France
Title: Composite nanofibers containing ruthenium nanoparticles stabilized by cyclodextrin polymers: synthesis, characterization and their application in heterogeneous catalysis

Biography
Dr. Sami Fadlallah has completed his PhD in Organometallic Chemistry and Homogeneous Catalysis by University of Lille-UCCS laboratory. Currently he is postdoctoral research associate in the field of polymer engineering and heterogeneous catalysis at UMET-Lille and UCCS-Artois laboratories
Abstract
The synthesis of functional nanofibers containing noble metal nanoparticles (NPs) is of growing interest, notably due to their application in different fields such as catalysis, medicine and sensing (1). These systems exhibit particular characteristics and they are defined by their high surface area of stabilized active metals. However, the size and homogeneous distribution of the incorporated metal NPs should be taken into consideration in order to reach the high performance and efficiency.
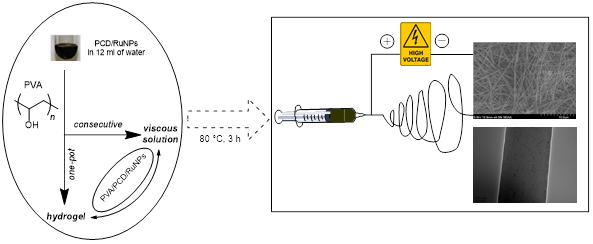
Herein, we report the synthesis of composite nanofibers of poly(vinyl alcohol) (PVA)/poly(citric acid-β-cyclodextrin) (PCD)/RuNPs. The new approach includes the preparation of a series of Ru colloidal nanosuspensions stabilized with PCD (2), then, the dissolution of PVA polymer which constituted the matrix of nanofibers during the electrospinning phase. Rheological study showed that the viscosity depends on both the molecular weight of PVA and amount of reducing agent; NaBH4. These heterogeneous catalysts were fully characterized by TEM, SEM, TGA and DSC. Moreover, the effect of NaBH4 (NaBO2) as a physical crosslinking agent was studied by heat treatment of electrospun nanofibers. Furthermore, the activity of the prepared catalysts was evaluated in the catalytic hydrogenation reactions in liquid phase.
References
- J. Xue, J. Xie, W. Liu, Y. Xia. Electrospun nanofibers: new concepts, materials and applications. Accounts of Chemical Research 50 (2017) 1976-1987.
- R. Herbois, S. Noel, B. Leger, S. Tilloy, S. Menuel, A. Addad, B. Martel, A. Ponchel, E. Monflier. Ruthenium-containing β-cyclodextrin polymer globules for the catalytic hydrogenation of biomass-derived furanic compounds. Green Chemistry 17 (2017) 2444-2454
Ismi Rajiani
STIA Dan Manajemen Kepelabuhan Barunawati Surabaya, Indonesia
Title: Which comes first: Technological or management innovation when disseminating the awareness of the green port urgency ?

Biography
Dr. Ismi Rajiani has completed his PhD in Management Science from University of Brawijaya Indonesia . He has worked as an assistant professor of International Business Management at STIA & Managemen Kepelabuhan Barunawati Surabaya, Indonesia, a college of business administration and port management under the auspices of Indonesia Port. He has published more than 50 papers in reputed journals including the Scopus and Web of Science Indexed ones and has been serving as an academic director of the school.
Abstract
Technological innovation with related notions such as product development, radical versus incremental innovation as well as diffusion and adoption has dominated innovation research. However, falling trade barriers, decreasing transaction costs, stagnating developed markets and overheating emerging markets are forcing firms to look for other areas in which to innovate as a means of gaining and maintaining competitive advantage. Management innovation is changing the nature of management within organizations by, for instance, adapting organizational structures, processes, and practices to generate a valuable source of competitive advantage. Scholars have started emphasizing that, in order to capture the full benefits of innovation, technological innovation needs to be combined with management innovation. Ironically, despite the recent surge in academic interest, management innovation remains an under-researched topic. To fill the gap, in this paper we will conceptualize management innovation to clarify understanding of management innovation, its underlying dimensions, antecedents, impact on performance as well as the contextual factors that affect management innovation. As most innovations are related to product development, this study highlights management practices as a process innovation in responding to the current trend. While there is a growing body of in-depth qualitative research that provides insight into the sequence of events that occur during process innovation, these highly context-dependent studies have not systematically analysed the organizational capabilities related to fuel management innovation. This way, Structural Equation Modelling (SEM) is used to spot the terrain for further study. Finally, the model is expected to support The Indonesian Port Cooperation in determining the policy to support the government agenda to reduce carbon emission as pledged in 2009 G20 Summit.

























